Ozone
is an unstable form of
molecular oxygen. About
90% of the ozone is found
in the upper stratosphere.
It forms a concentrated
band, roughly between
19 and 23 km above the
earth’s surface,
and acts as a shield
absorbing ultraviolet
radiation, especially
UV-B.
Man-made chemicals such as chlorofluorocarbons (CFCs) released into the atmosphere
start chemical reactions that destroy the ozone layer.
The
picture alongside
shows the hole
in the ozone
layer (area marked
grey) over the
Antarctic. The
affected area
depicted is approximately
28 Mio km2 or
8.5 times the
size of the Indian
continent!! It
will require
many decades
(scientists say
50 years
or more) to repair
the damage, even
with the Ozone
Depleting Substances
phased out.
Protecting
the Ozone Layer
The first international agreement to protect the ozone layer was the Vienna
Convention on the Protection of the Ozone layer. This was followed by the Montreal
Protocol on Substances that Deplete the Ozone layer in 1987. The Montreal
Protocol was substantially revised four times, the latest being in 1999 (Beijing).
The Montreal Protocol stipulates that the production and consumption of compounds
that deplete the ozone layer like chlorofluorocarbons (CFCs), halons, carbon
tetrachloride, and methyl chloroform--are to be phased out by 2010 (2005 for
methyl chloroform). |
Total
ozone (DU)/ Ozone total
(UD), 2006/09/25
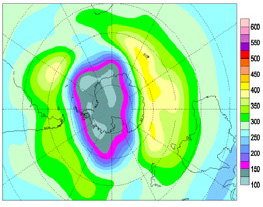
DU
- Dobson unit
Ozone Hole Antarctica
25 September 2006
|
Ozone
Depletion has a major
impact on
health,
agricultural products,
animal and
plant life as well as
materials.
WHAT IS THE OZONE LAYER?
Ozone is a reactive gas
consisting of three
oxygen atoms, formed naturally in the atmosphere
by the association of molecular oxygen (O2) and
atomic oxygen (O). Most ozone (about 90%)
resides in the stratosphere (a layer of the
atmosphere between 10 to 40 km above the
earth), where it acts as a shield to protect
the Earth's surface from the sun's harmful
ultraviolet radiation (UV-B), filtering out the
high energy radiation below 0.29 µm and allowing
only a small amount to reach the Earth’s
surface. The high concentration of ozone in this
part of the atmosphere is commonly known as the
ozone layer.
THE DEPLETING OZONE LAYER
AND THE OZONE HOLE
In recent years, data collected
in the upper
atmosphere has shown a general thinning of the
ozone layer over most of the globe. More
dramatic damage occurs over Antarctica each
spring when the ozone hole forms. This depletion
in the ozone layer is far beyond seasonal
variations, and the natural balance is not being
restored. Research has revealed that man-made
chemicals released into the air are contributing
to the depletion of the ozone layer.
The
consequences of the depleting
ozone layer are severe – the increased levels of harmful
UV-B radiation entering the Earth’s atmosphere
impact adversely on human, animal and plant
life on the planet.
The
harmful consequences include:
a)
Increased incidence
of skin cancer, eye
diseases and weakening
of the immune system
in humans.
b)
Damage to marine life, particularly planktons,
leading to disruption in the food chain and loss
of bio-diversity.
c) Increased incidence of eye and skin disease
amongst the animal population on the planet.
d)
Reduction in food production as a result of
damage to food crops by the UV-B radiation as
well as loss of plant bio-diversity.
OZONE DEPLETING SUBSTANCES
(ODS)
Research has revealed that there are
several chemicals such as chlorofluorocarbons,
halons and methyl bromide that contribute
to ozone depletion. In the early 1930s
when CFCs were first discovered,their
non-toxic, non-flammable, and non-reactive
properties made them ideal for many
industrial and domestic applications
like coolants for commercial and home
refrigeration units, aerosol propellants,
electronic cleaning solvents, and
blowing agents. Chlorofluorocarbons
were in great demand and produced
in abundant quantities. It was only
in 1973 that scientists discovered
that the chlorine found in CFCs causes
ozone destruction.When CFC molecules
drift into the atmosphere, the UV-B
and UV-C radiation from the sun releases
their chlorine atoms. Complex chemical
reactions in the atmosphere result
in the formation of chlorine monoxide,
which reacts with the ozone molecule
to form oxygen and regenerates more
chlorine atoms that carry on converting
the ozone molecules. Each chlorine
atom can destroy as many as 100,000
ozone molecules over 100 years. Thus,
even a small amount of CFCs can cause
tremendous damage to the ozone layer.
THE MONTREAL PROTOCOL
Faced with the strong possibility
that CFCs and similar compounds could
cause serious ozone depletion, in
1987, policy makers from around the
world signed an international treaty,
the Montreal Protocol on Substances
That Deplete the
Ozone Layer. According to this protocol,
countries would phase out CFCs and
other ODS as per a given schedule,
with a complete halt by 2010. More
than 187 countries are signatories
to the Montreal Protocol.
Under the Protocol, industrialized
nations have rapidly eliminated most
ozone depleting substances. Developing
countries are following suit, with
critical assistance from the Protocol's
Multilateral Fund, which has already
committed over US $ 1.5 billion to
assist developing countries in the
difficult transition to ozone-friendly
substances.
For further information about the
NCCoPP project and press related issues,
please write to
|
|





