|
|
|
OZONE SHIELD IN DANGER |
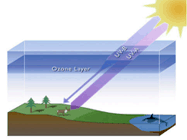
Our earth is enveloped in a protective shield
of ozone which prevents harmful ultraviolet rays
from reaching the Earth. This shield is known as the ozone
layer.
|
 THE OZONE LAYER
THE OZONE LAYER
Ozone molecules consist of three oxygen atoms as signified
by the chemical symbol O 3. Ozone molecules exist naturally in the upper levels of the atmosphere known as
stratosphere that stretches from about 15 to 55 km above the Earth's surface. At this altitude both ozone
and oxygen coexist in a dynamic equilibrium maintained by high energetic UV radiation from the sun.
In fact, the amount of ozone in the stratosphere is frighteningly small.
The highest concentration of ozone, found at an altitude of 20 to 25 km, is only 10 parts per million (ppm).
If we could gather all stratospheric ozone on the ground under atmospheric conditions, it would amount to a
layer no thicker than a mere 3 mm. To simplify, we imagine the ozone layer as a thin curtain around our
Earth's atmosphere. However, in reality ozone is scattered over the stratosphere in low concentrations.
|
WHAT THE
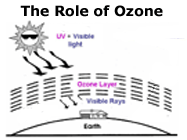
OZONE LAYER DOESDespite its small quantity and low concentration, ozone plays a critical role in
chemical and biological processes on earth. Ozone absorbs the sunlight's high-energy
ultraviolet radiation (UV-B) of wavelengths of 280 to 320 nm. Thus the ozone layer functions
like a shield protecting life on our planet from harmful UV-B. A decrease in the ozone concentration
results in greater quantities of UV-B reaching the earth's surface with
damaging effects on all biological systems. This poses a serious threat to life on Earth as we know it.
|
DISCOVERY
OF THE OZONE HOLE
Scientific studies conducted since the late 1970s confirmed that the concentration of ozone in the
stratosphere is dropping. The discovery of an annual formation of an 'ozone hole' over Antarctica,
a zone with significantly less ozone than usual, provided further evidence of this phenomenon. Successive
observations showed an enlargement of this hole over time and an increase in the UV-B radiation reaching
the surface of our planet.
This discovery, along with evidence that ozone is being lost at nearly all latitudes outside the tropics,
has prompted much research into the causes of this depletion and the biological effects of increased
exposure to UV-B rays. Today it is scientifically established that man-made chemicals are the source of
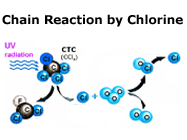
ozone depletion. The culprits have been tracked down to some compounds containing Chlorine and Bromine.
The culprit compounds are technically known as ozone depleting substances (ODS).
|
Once released, ODS rise slowly up to the stratosphere and interact with ozone.
In the process they break up ozone molecules, leaving behind just oxygen (O2).
ODS molecules are capable of repeating their work of destruction several thousand times before
decay disarms their aggressive potential, destroying thousands of ozone molecules during their life span.
The natural rate of ozone regeneration is much slower than its rapid destruction.
Thus the natural balance is disturbed and the ozone concentration continues to decrease.
|
 |
|